ClariTest® Core TESTING
To learn more about our ClariTest® Core testing, please visit our GenPath website.
Learn More
ClariTest® Core is a non-invasive prenatal screen (NIPS) that identifies the risk for fetal chromosomal abnormalities. ClariTest Core can be performed as early as 10 weeks gestation from a simple blood draw. Depending on the patient’s location, the courier networks, weather-related delays, etc., it may take a few days for us to receive the specimen. Once it arrives, results are usually release within five to seven days. ClariTest® Core can be used to screen singleton and egg donor/IVF pregnancies for the common trisomies, sex chromosome aneuploidies and 22q11.2 microdeletions. Twin gestations can be screened for the common trisomies and for presence of the Y chromosome.
CONDITIONS SCREENED FOR WITH CLARITEST® CORE
| Trisomy 21 (Down syndrome)1 | Trisomy 18 (Edward syndrome)3 | Trisomy 13 (Patau syndrome)4 |
| Monosomy X (Turner syndrome)5 | XXY (Klinefelter syndrome)7 | XXX (Trisomy X)8 |
| XYY (Jacob syndrome)9 | XXYY10 | 22q11.2 syndrome (DiGeorge syndrome)11* |
For more information related to conditions or services, click here.
ClariTest Core offers the option to add screening for 22q11.2 microdeletion syndrome, also known as DiGeorge syndrome. DiGeorge syndrome is an autosomal dominant condition, and is the second most common genetic cause of heart defects and developmental delay, after Down syndrome.1 Unlike trisomies, maternal age does not increase the chance for 22q11.2 microdeletion, and more than 90% of affected individuals have no family history of the condition. 2
22q11.2 microdeletion affects as many as 1 in 1,000 pregnancies.3
The performance of the ClariTest® Core prenatal screen for 22q11.2 microdeletion has been evaluated in the largest 22q11.2 validation study with over 1,900 hundred cases, 129 with confirmed deletions.4
ACMG recommends informing all pregnant women, including women at low or average risk, that NIPS is the most sensitive screening option for trisomy 13, 18, and 21.5 ACOG and SMFM confirm that cfDNA screening (NIPS) is an appropriate choice of testing for all pregnant women, regardless of age or risk and that NIPS is the most sensitive and specific screening test for common aneuploidies.16
ClariTest Core utilizes the DANSRTM platform and FORTETM algorithm, the same core technology as harmony®, which is the most widely studied cell-free DNA methodology, with over 60 peer-reviewed publications. The studies demonstrated exceptional performance in singleton and twin pregnancies and in women of any age or risk category6-13 and established the superior accuracy and reproducibility of the platform for fetal fraction assessment.14
| Disorder | N | Sensitivity | Specificity |
| Trisomy 212 | 23,155 | 99.3% (418/421) | 99.96% (22,724/22,734) |
| Trisomy 182 | 22,399 | 97.4% (147/151) | 99.98% (22,243/22,248) |
| Trisomy 132 | 14,243 | 93.8% (30/32) | 99.98% (14,208/14,211) |
| Monosomy X3 | 1,381 | 94.3% (82/87) | 99.8% (1,291/1,294) |
| 22q11.2 Microdeletion4 | 1,953 | 75.2% (97/129) | 99.6% (1,816/1,824) |
| XXX/XXY/XYY/XXYY3 | Other sex aneuploidies will be reported if detected. Limited data of these more rare aneuploidies preclude performance calculations. |
Fetal Sex: Accuracy for fetal sex (male or female) is >99%3
ClariTest Core utilizes microarray quantitation and a proprietary, targeted technology that includes both the DANSR assay and the FORTE algorithm to provide exceptionally accurate results.

Results will be reported as high- or low-risk:
![]() High Risk: Positive Predictive Value (PPV) will be reported if result is high risk for trisomy 21, 18, 13, monosomy X, or a 22q11.2 microdeletion in singletons
High Risk: Positive Predictive Value (PPV) will be reported if result is high risk for trisomy 21, 18, 13, monosomy X, or a 22q11.2 microdeletion in singletons
![]() Low Risk: Negative Predictive Value (NPV) >99% for all conditions and is reported for low-risk singleton pregnancies
Low Risk: Negative Predictive Value (NPV) >99% for all conditions and is reported for low-risk singleton pregnancies
ClariTest Core is a screening test. Patients receiving a high risk result are advised to seek genetic counseling, as well as to discuss diagnostic testing options. Decisions regarding the pregnancy should NOT be made based on ClariTest Core results only.
BioReference® Health and its division GenPath, is an in-network provider for all major national and many regional health plans, increasing your access to ClariTest Core non-invasive prenatal screening.
References
1) McDonald-McGinn DM et al. Nat Rev Dis Primers. 2015;1:15071. doi: 10.1038/nrdp.2015.71.
2) McDonald-McGinn DM et al. Genet Med. 2001; 3(1):23-9.
3) Grati FR et al. Prenat Diagn. 2015;35(8):801-809.
4) Schmid M et al. Fetal Diagn Ther. 2018;44(4):299-304.
5) Gregg AR et al. Genet Med. 2016;18(10):1056-65.
6) Stokowski et al. Prenat Diagn. 2015;35(12):1243-6.
7) Jones K et al. Ultrasound Obstet Gynecol. 2018 51:274-277.
8) Norton ME et al. Am J Obstet Gynecol 2012;207:137.e1-8.
9) Nicolaides KH et al. Am J Obstet Gynecol.2012 Nov;207(5):374.e1-6.
10) Gil MM et al. Ultrasound Obstet Gynecol. 2019;53(6):734–742.
11) Gil MM et al. Fetal Diagn Ther. 2014;35:204-11.
12) Jones K et al. Ultrasound Obstet Gynecol. 2018;51(2): 275–6.
13) Schmid M et al. Ultrasound Obstet Gynecol. 2018;51(6):813-17.
14) Sparks AB et al. Am J Obstet Gynecol. 2012;206:319.e1-9.
15) Juneau K et al. Fetal Diagn Ther. 2014;36(4):282-6.
16) Rose NC et al. Am J Obstet Gynecol. 2020;136(4):e48-69.
To learn more about our ClariTest® Core testing, please visit our GenPath website.
Learn MoreEste sitio web (y las páginas contenidas) estan desactivado. Si se necesita asistencia en español u otro idioma, por favor llame sin cargas a 800 229 5227.
| Code | SST | 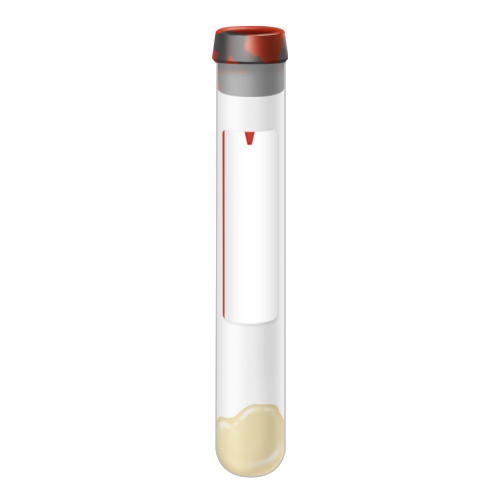 |
|---|---|---|
| Name | SST Tube | |
| Code | BML | 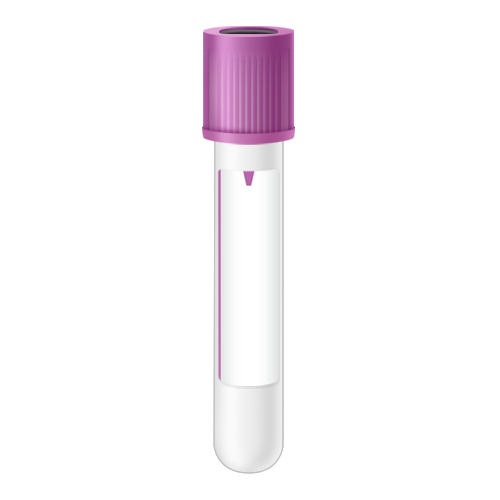 |
| Name | Bone Marrow - Lavender Top | |
| Code | LAV |  |
| Name | Lavender top- EDTA | |
| Code | FLUL |  |
| Name | Fluid Lavender (source required) | |
| Code | TAN |  |
| Name | Tan Top | |
| Code | YEL |  |
| Name | Yellow top- ACD | |
| Code | YELS |  |
| Name | Yellow top- SPS | |
| Code | RBP | 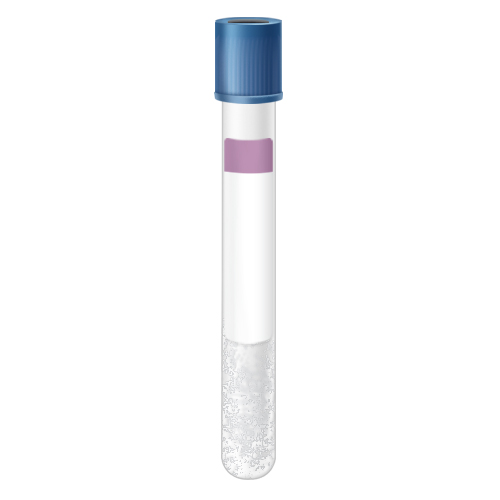 |
| Name | Blue-Royal Blue Top K2 EDTA Plasma(*LAVENDER LINE) | |
| Code | RBS | 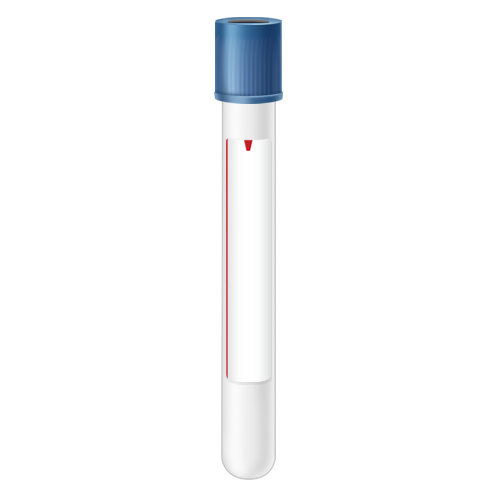 |
| Name | Blue-Royal Blue Trace Element Serum (**RED LINE**) | |
| Code | URB |  |
| Name | Blue-Royal Urine | |
| Code | GRY |  |
| Name | Grey top- Sodium Fluoride | |
| Code | BMG |  |
| Name | Bone Marrow - Green Top | |
| Code | UG |  |
| Name | Green top urine | |
| Code | UGTP |  |
| Name | Green top urine (preservative) | |
| Code | FLUG |  |
| Name | Fluid Green Top | |
| Code | QFT |  |
| Name | Green Top-Lithium Heparin | |
| Code | FLUR |  |
| Name | Fluid Red Top | |
| Code | LTB |  |
| Name | Light blue top | |
| Code | PPT |  |
| Name | White Top (PPT) | |
| Code | ACDNA |  |
| Name | Ariosa Cell-Free DNA (White TopTube) | |
| Code | TP |  |
| Name | ThinPrep Vial | |
| Code | SPTH |  |
| Name | Surepath Vial | |
| Code | O&P |  |
| Name | O&P Kit (Total Fix) | |
| Code | U24 |  |
| Name | Urine Container - 24hr | |
| Code | UA |  |
| Name | Urine Urinalysis Tube - Yellow | |
| Code | CUL |  |
| Name | Swab-Bacterial Culture | |
| Code | ES |  |
| Name | Swab-E | |
| Code | APT |  |
| Name | Swab-Aptima Genprobe | |
| Code | UGP |  |
| Name | Urine Container-Genprobe-Aptima | |
| Code | BDA |  |
| Name | Affirm(BD) VPIII | |
| Code | RED |  |
| Name | Red Top Plain | |
| Code | UGY |  |
| Name | Urine Tube - Grey Top | |