Bloomberg
There’s no hard evidence that antibodies to the virus that causes Covid-19 provide immunity
Covid-19 antibody tests are suddenly everywhere.
A West Palm Beach spa that normally offers Botox and collagen treatments touts tests on Instagram. A New York City urgent-care chain emails patients that the tests are available, though “not 100% accurate.” A chiropractic clinic in Minnesota promises its tests will provide “fast and accurate peace of mind.”
Antibody tests, which look for markers in the blood that indicate exposure to the novel coronavirus, have become widely available in the U.S. in the past month, even as a debate rages about their medical value and how best to interpret the results. For policy makers and health experts, the tests could offer insights into the virus’s prevalence and spread the country currently lacks. But for individuals concerned about their own health, the benefits are less clear.

Experts believe antibodies probably convey some level of protection against the virus, but they don’t have any solid proof yet. Nor do they know how long any immunity may last. Even so, thanks to an initially permissive stance by the U.S. Food and Drug Administration, hundreds of new tests are now on the market. And the proliferation has led to questions about their accuracy.
“The problem with the tests is it’s not clear who needs them or how to feel about the results,” said George Liakeas, a physician who serves as medical director of Lexington Medical Associates in New York City. But patients are asking for them anyway, posing “a unique predicament.”
Labs that offer antibody tests say they can offer valuable validation to people who suspect they may have had Covid-19, eventually got better but were never tested. The tests also can identify donors of plasma to help severely ill patients. Yet they also carry risks, including exposure to the virus itself while out of the house, as well as false-positive readings. Experts worry these could prompt people to take fewer public-health precautions, including social distancing and mask-wearing.
The risks haven’t stopped people from taking the tests. Some businesses that stayed open the past two months, such as factories and banks, are encouraging employees to get them as a tool to screen those with antibodies and thus deemed safer to be at work. Commercial lab giant Quest Diagnostics Inc. has performed about 620,000 antibody tests since it introduced them on April 21, the company said.
Juanita Mora, an allergist-immunologist who practices in Chicago and treats many patients in the Latino community, which has been particularly affected by the virus. Mora sais she has seen a number of local employers, from banks and car dealerships to a hot-dog manufacturer and tortilla factory, use antibody testing to screen employees returning to work. Mora herself uses the results in conjunction with a patient’s medical history to make decisions about infection risk, including whether to go back to their job, and for patients who were exposed or sick and have been self-isolating from their families.
Antibody testing “will help us walk people back into a new normal,” Mora said. “I think it’s actually going to be a key test in controlling this pandemic.”
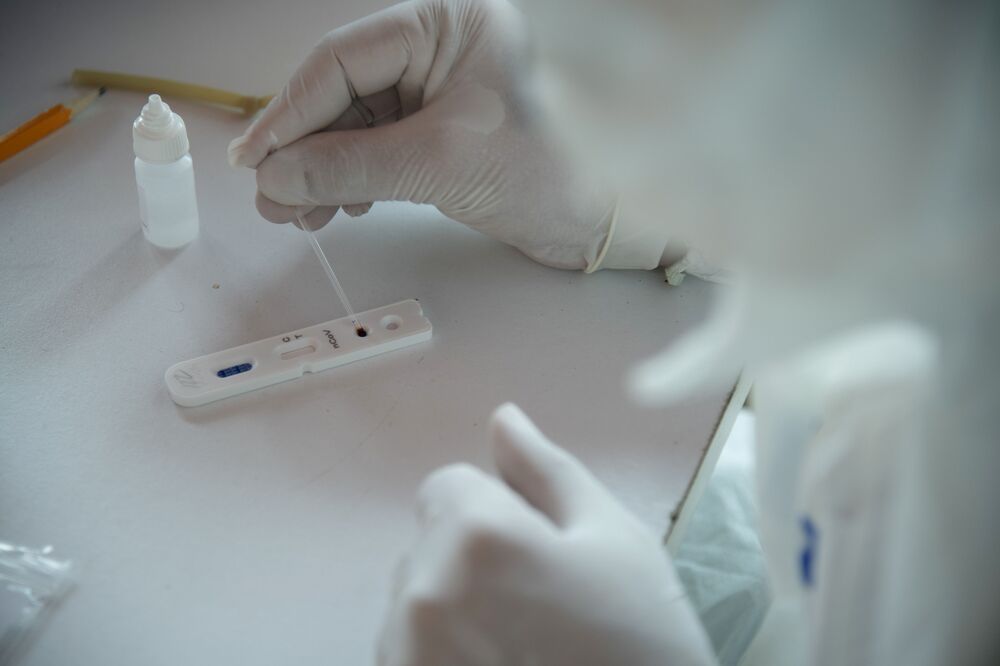
Within an hour of emailing patients in late April about test availability, Lexington Medical Associates was “overwhelmed” with inquiries, Liakeas said. About 200 have participated so far, about a quarter of whom tested positive for antibodies. Liakeas, who was hospitalized with Covid-19 in March and is still recovering, recenly took the test and got a positive result.
In New York City, the commercial BioReference Laboratories Inc. will begin providing free antibody tests to the general public on May 13 as part of a partnership with New York City Health and Hospital Corp. aimed at testing several hundred thousand individuals, said Jon Cohen, a physician who serves as BioReference’s executive chairman.
Despite the questions about what test findings mean for the future, Cohen says he believes the results carry a psychological impact for people who take them. He himself got tested last week, as part of a free offering for BioReference employees, and the result came back negative.
In announcing stronger oversight over antibody tests last week, the Food and Drug Administration said it is taking action against kit makers with allegedly false or inaccurate claims. These include a “concerning number” of commercially sold antibody tests being “inappropriately” promoted, including as a diagnostic test, according to the FDA.
Quest has begun contacting almost two dozen partners that resell its testing services, asking them to discontinue offering the tests after seeing information on the partners’ websites that “may not have met our bar for quality,” spokeswoman Wendy Bost said in an interview.
“We have concerns about making sure that the patients have access to a provider consult and making sure that the information for how the tests can be used is aligned with our understanding of the value of these tests,” Bost said. “Covid is a new world, and it’s creating new scenarios for us to address.”
Positive antibody-test results didn’t give Binita Riffat, a 22-year-old urgent-care worker in New York City, all the answers she was looking for. Following weeks of dealing with patients who had Covid-like symptoms, she now worries she may have inadvertently brought the virus home to her parents and brothers.
After hearing Georgia Governor Brian Kemp say antibody testing was available, Hayden Barnes, a developer at a tech company who lives in Columbus, got tested on May 8. He hoped it could reveal whether an illness he and his daughter had in March was Covid-19.
He also didn’t get the result he had wanted. “Knowing that I was exposed and didn’t die would be a bit of a relief,” he said. Two days later, though, the test came back negative.
— With assistance by Kristen V Brown
Click here for original post on Bloomberg